In my February 2020 progress update, I mentioned a ‘potential cure’ that had been discovered in January, by researchers at Cardiff University. And this post is designed to answer the question, can the Cardiff killer T cell cure cancer?
Before I can start answering that, I need to vastly oversimplify a few things, starting with blood.
For the purposes of this post, blood can be thought of as having two types of cells, suspended in a liquid called plasma. The types of blood cells are:
- Red blood cells, which carry oxygen around the body, and;
- White blood cells, which are part of the body’s immune system and protect the body from infection
Of these two, we’re interested in the white blood cells, which are collectively known as leukocytes.
Brace yourself, we’re going in…
Leukocytes can be divided into five main types:
Of these, the type of cells we’re interested in are the Lymphocytes. But we’ve got to dig a bit deeper yet. Because, you’ve guessed it; there are a variety of types of Lymphocyte:
And, despite the Natural Killer cells sounding the coolest, by far, we’ll be looking at the T Cells.
The ‘T’ cell is so called because it develops in the Thymus gland. And even this sub-type of white blood cell, has a couple of varieties. These include the CD8+ T cells, which are cytotoxic and known as the ‘Killer T cells’…
We made it!
Being cytotoxic means that killer T cells can actually be toxic to other cells. As such, one of the functions of killer T cells is immune-mediated cell death. This means that killer T cells are able to directly kill pathogen-infected cells. Killer T cells can also, and this is the really good bit; directly kill cancer cells.
The way the killer T cells do this, is by triggering apoptosis in the target cell. Apoptosis is a normal and natural process that cells go through when they stop working properly. It’s a programmed form of cellular suicide. Most cells will do this of their own accord. The remains of the former cell are then cleaned up by white blood cells and a replacement cell will be formed by mitosis.
Sometimes, however, cells are not able to complete apoptosis on their own, which is when the killer T cells take over. If a cell isn’t operating properly, a killer T cell rocks up and forces the faulty cell to self destruct. It’s pretty horrific, when you think about it. Still, that’s why it’s best not to anthropomorphise things.
Besides, apoptosis is vastly preferable to necrosis, which is what would happen otherwise. If cell necrosis occurs, then the dead cell starts decomposing where it is. The dead cell basically starts rotting, which then spreads to neighbouring cells. An example of this is gangrene. And, suddenly, T cell induced apoptosis doesn’t sound so bad.
So, we’re good. Killer T cells force cancer cells into apoptosis, and everyone’s a winner…
Except, that in a lot of cases, the cancer is so sneaky that it can hide from the killer T cells. Consequently, tumours become malignant and metastases can form.
Which brings us to the Cardiff killer T cell.
The study was published on 20th January 2020, in Nature Immunology. To gain access to the full study would cost a reasonable chunk of money, for something I largely wouldn’t understand anyway. Fortunately, the SciShow YouTube channel did a video on it, which is far more accessible!
The most uniquely interesting thing that the SciShow video reveals about the discovery of the Cardiff killer T cells impact on cancer, was that it was an accident! The study was actually looking at ways to attack bacteria, using killer T cells. Or, more specifically, they were looking for a killer T cell that could identify lots of different types of bacteria, hidden within their host cells. Because, typically, any given killer T cell can only identify one type of bacterial infection.
To conduct the test, the team was using a type of cancer cell that happens to be easy to infect with bacteria. Then they were letting the killer T cells loose among the cancer cells. Not to try and kill the cancer cells, themselves, just the cancer cells infected with the bacteria. What they found was that one of the strains of killer T cell they tested, simply slaughtered every cancer cell it came in contact with. Which was a complete disaster, in terms of the experiment.
But absolutely amazing news if you’re interested in a cure for cancer!
Thankfully, the researchers recognised this… Honestly, I shudder when I think that this could just have been scrapped as a bad strain of killer T cells, for the experiment, and the researchers just moved on. Brrrrr!
But they didn’t.
Which is why we now need to talk about CAR T Immunotherapy.
One of the more successful recent developments in immunotherapy is Adoptive Cell Transfer (ACT). This is the process of collecting a patient’s own immune cells, tweaking them outside the body, and then unleashing them back into the patient’s body. At which point, they can ‘see’ the cancer cells and start giving them a good kicking. As you can probably imagine, this process is not quick, easy or cheap. It has to be specifically tailored to each individual patient.
Of the various ACT options, the one that seems to have the most potential is CAR T cell Therapy. Chimeric Antigen Receptor (CAR) T cells are genetically engineered to create an artificial receptor, on the surface of the T cell, that can ‘see’ the cancerous cells. Again, this is not quick, easy or cheap but it does allow for the development of receptors that can be used on multiple patients.
So far, CAR T cell therapies are still largely in the clinical trial phase. That said, some CAR T cell therapies are available on the NHS for leukemia and lymphoma, particularly in children. It makes sense to focus on blood cancers, to start with, because surgery is so seldom an option. For more information on CAR T cells, see the National Cancer Institute page.
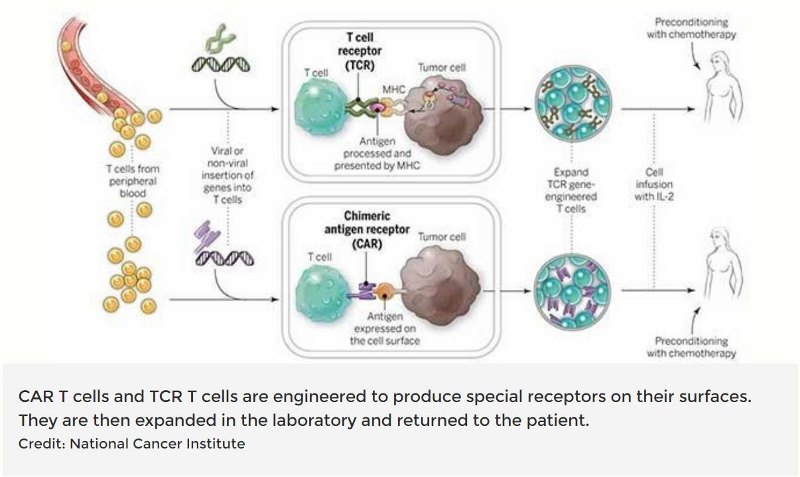
Right, so, while CAR T cell therapies have the potential to be very effective, they tend to be very specific to the patient and are neither cheap nor easy.
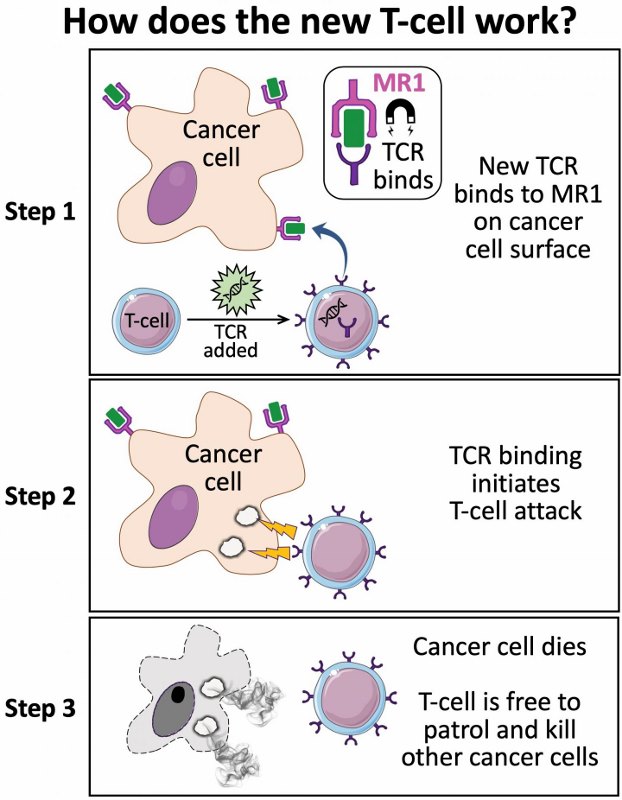
The Cardiff killer T cell discovery has the potential to change all this.
Because, after the Cardiff killer T cell wiped out all the cells of the original type of cancer, the researchers repeated the experiment on different types of cancer cells. And the Cardiff killer T cells worked on virtually all of them. The Cardiff killer T cell had receptors that could ‘see’ all of the types of cancer cells and killed the lot. At the same time, ignoring the healthy cells.
Let me just repeat that: the Cardiff killer T cells kill all cancer cells, while leaving all the healthy cells alone.
Wow!
The researchers then went on to use CRISPR to identify that the protein responsible for all this is MR1. Oddly enough, the MR1 protein can be found on all cells, not just cancerous ones. The MR1 protein presents information about the state of the cells, which can then be ‘seen’ by receptors on T cells. And, when a receptor on a Cardiff killer T cell ‘sees’ the information presented by the MR1 protein on a cancerous cell, it then instructs said cell to kill itself.
As you’d expect, this has been shown to work in test tubes (in vitro). But it’s also been shown to work on mice, using human cells lines (in vivo). Best of all, these Cardiff killer T cells have been shown to work in test patients’ cancers. On a number of people and a number of cancer types. A precursor to clinical trials, if you will.
And clinical trials are expected to be started by the end of the year…
It’s impossible to emphasise how big a deal this really is!
Because all of us have the MR1 protein on our cells. So, while this remains a CAR T cell therapy, the Cardiff Killer T cells can be used for everyone, regardless of what the cancer type is. Meaning, if this comes off, it will be a ‘Universal’ treatment, that works for everyone.
An actual cure for cancer…

Photo by Jason Leung on Unsplash
To put it in the words of Professor Awen Gallimore, of Cardiff University’s division of infection and immunity and cancer immunology,
“If this transformative new finding holds up, it will lay the foundation for a ‘universal’ T-cell medicine, mitigating against the tremendous costs associated with the identification, generation and manufacture of personalised T-cells.”
https://www.cardiff.ac.uk/news/view/1749599-discovery-of-new-t-cell-raises-prospect-of-universal-cancer-therapy
In an ideal world, in a couple of years, anyone diagnosed with cancer gets given a dose of Cardiff killer T cells and then gets back on with their life.
So, do we live in this ‘ideal world’?
Only time will tell.
In reality, there’s along way to go. Moving forward, Cardiff University will be working in collaboration with Ervaxx to develop, ‘Immunotherapeutics targeting Dark Antigens’. As quoted from the Ervaxx news release. ‘Dark Antigens’, by the way, is a trade marked term. I’m suddenly feeling a bit more nervous about all this…
Still, you’ve got to trust that Cardiff University will have made the best choice for the development of their killer T cells. Especially as, if this goes right, there are going to be Nobel prizes involved at some point. Besides, Dr Andrew Sewell, of Cardiff University, is on Ervaxx’s Scientific Advisory Board.
The difficulty, now, is to not get too excited by all this. Not least because of all the lauded ‘universal cancer cures’ that have failed to become reality, over the years. And even if everything goes perfectly, it’ll be a matter of years before a treatment is approved. Although clinical trials are programmed to start within a year, for those of us who might need them.
But it’s difficult not to get excited. Because this seems really promising.
So, back to the issue of whether or not the Cardiff killer T cell could cure cancer…
You know what? It really might.
2 thoughts on “Can The Cardiff Killer T Cell Cure Cancer?”
So is this new approach an improved version of CAR-T cell therapy? The article wasn’t clear about this.
Hi Phil,
Yes, this new approach is part of the CAR-T cell therapy family. It just has the potential to appear different because of the much lower levels of engineering involved. In theory, one generic batch of this particular CAR-T cell therapy will fit all.
All the best,
Paul