In January 2019, Dan Aridor, of the Israeli company AEBi, is quoted as saying, “We believe we will offer in a year’s time a complete cure for cancer.” The cure that he was talking about is called Multi-Target Toxin, or MuTaTo, for short. Well, it’s been more than a year, so let’s check back to see whether MuTaTo can cure cancer.
The mass excitement started when the Jerusalem Post reported on a development made by Accelerated Evolution Biotechnologies (AEBi). The report included some remarkable soundbites, including:
“Our cancer cure will be effective from day one, will last a duration of a few weeks and will have no or minimal side-effects at a much lower cost than most other treatments on the market,” Aridor said. “Our solution will be both generic and personal.”
https://www.jpost.com/HEALTH-SCIENCE/A-cure-for-cancer-Israeli-scientists-say-they-think-they-found-one-578939
As well as:
Our results are consistent and repeatable.
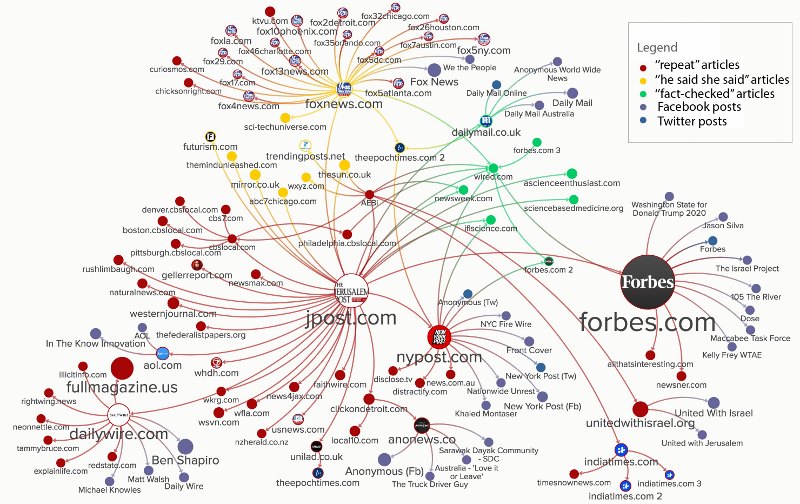
As you would expect, such exciting and quotable declarations flew around the world at the speed of Retweet. This meant that the news was repeated, far and wide, including by some very sizeable news organisations. As such, it got on to sites like Quora, where cancer patients were desperately seeking confirmation that it was true.
There was, however, a lamentable lack of fact checking in the vast majority of reports about this story:
Source: Health Feedback
A compounding factor was that another Israeli company, IceCure, made a cancer related announcement at around the same time. IceCure was marketing their latest development in cryoablation, called ProSense. IceCure has a solid record in the field of cryoablation and ProSense was an improvement in their existing range. There was a lot of hyperbole in the product launch, because that’s marketing.
It seems that the established reliability of IceCure got tangled up with the announcement coming out of AEBi, meaning that the latter’s claims were taken very seriously indeed. I wrote a post about IceCure and ProSense back in August 2019, if you want to read more about that.
So then, what is involved with MuTaTo and can it cure cancer?
MuTaTo appears to be a form of immunotherapy, with an approach based on peptides instead of antibodies. The Jerusalem Post article then linked this bit of information back to the 2018 Nobel Prize winner for Chemistry. Despite said Nobel win being for work on antibodies, the very thing that MuTaTo was moving away from…
To my mind this is an… interesting… choice of journalism.
The piece then goes into more detail of what’s involved. It is made clear that, like a lot of immunotherapy treatments, MuTaTo would have to be individually tailored to each patient.
In the last paragraph of the article, it is revealed that, “so far, the company has concluded its first exploratory mice experiment.”
Which is followed up with:
AEBi is on the cusp of beginning a round of clinical trials which could be completed within a few years and would make the treatment available in specific cases.
https://www.jpost.com/HEALTH-SCIENCE/A-cure-for-cancer-Israeli-scientists-say-they-think-they-found-one-578939

Photo by Nathan Dumlao on Unsplash
This announcement sounds utterly incompatible with the start of the article, which had this as a title:
A cure for cancer? Israeli scientists may have found one
And an opening sentence of:
A small team of Israeli scientists think they might have found the first complete cure for cancer.
Immediately under which is written the quote from Dr Aridor, stating that he believes he can offer a complete cure for cancer within a year.
In all honesty, it seems to me that Dr Aridor is talking about a concept. The majority of the article, however, makes it appear as if he’s talking about a physical cure. And, okay, the article clarifies the situation in the final paragraph, where it is made known that a usable treatment is still some years away. But how many people have actually worked their way through all the scientific and medical text to get to the final paragraph?
Not enough!
So, what has AEBi got to say about all this? How has it impacted on the company?
Well, it seems that AEBi is still working on MuTaTo. Which is definitely a good thing. Any attempt to find a cure for cancer should be lauded.
The AEBi site includes a link to a Pharmafile article from Autumn 2018 entitled, “MuTaTo: A novel concept for curing cancer“. Pharmafile has been established in the field of pharmaceuticals since 1986. It is a reputable organisation that allowed AEBi to showcase the MuTaTo concept. This is, by no means, an endorsement from Pharmafile but it is evidence that the MuTaTo concept is a valid one.
Far more recently, and since the end of the fabled year in which the cure would appear, AEBi has published a paper about MuTaTo. It was released in February 2020 by the Journal of Cancer Therapy (JCT), through Scientific Research Publishers (SCIRP). The paper is entitled, MuTaTo© – A Novel Concept for Curing Cancer…
SCIRP describes itself as, “one of the largest Open Access journal publishers.”

Photo by Alex Holyoake on Unsplash
Wikipedia, on the other hand, has the following to say of SCIRP:
Scientific Research Publishing (SCIRP) is an academic publisher of presumably peer-reviewed open-access electronic journals, conference proceedings, and scientific anthologies of questionable quality. Although it has an address in southern California, in reality it is a “Chinese operation”… In the Norwegian Scientific Index the publisher and all its journals have a rating of 0 (non-academic).
https://en.wikipedia.org/wiki/Scientific_Research_Publishing
Without reading it in detail, the February 2020 paper seems to say much the same as the autumn 2018 article. MuTaTo uses a multi-targeted approach against the mutagenic tendencies of cancer, and outperforms mono-target approaches. MuTaTo has also been successfully tested in vitro and in vivo, which is to say, in test tubes and mice.
To my mind, this is not a promising development. In the 15 month gap between the two publications, it does not appear that there has been a great deal of progress. Worse still, the second publication has been presented as a scientific paper on a dubious platform.
And as I’ve said before, and will no doubt say again, a ‘cure’ that only works in test tubes is no cure at all. If you put some cancer cells in a test tube and add vodka, for example, the cancer cells will die. This does not mean that an I.V. of vodka will cure your cancer. Well, technically, the cancer will die when the patient does, but I don’t think we can count that as an effective cure! And what is true of vodka, is true of bleach, lemon juice, etc. It is also true of MuTaTo…
Mice studies is a definite step in the right direction. This is where you demonstrate that the in vitro ‘cures’ of vodka, bleach and lemon juice don’t work in vivo. A bunch of dead mice rule out the use of vodka, bleach and lemon juice on people. MuTaTo, however, resulted in living mice and dead cancer tissues, meaning it might still be a cure for cancer in humans.
But the only way to know if a ‘potential’ cure can become an ‘actual’ cure in humans, is with clinical trials. And, as to whether there have yet been clinical trials, please see a screen grab of the ‘Contact Us’ section of the AEBi website:

http://www.aebi-bio.com/
So, where does that leave us?
Clearly, in answer to the question, ‘is MuTaTo a cure for cancer’, the answer is a resounding, no.
But what about the future? Is it possible that MuTaTo may yet turn out to be the cure for cancer, as suggested?
Of all the organisation that reported on the MuTaTo hype in 2019, only Forbes has followed it up a year later. And Forbes weren’t messing around with their timings, either. Their original article was dated Jan 29, 2019. Their follow-up piece was released on Jan 20, 2020. Forbes gave the claims about MuTaTo one day’s grace!
I have to admit, I like Forbes’ style!
You will also notice that Forbes is one of the few organisations to appear in the green, ‘fact-checked’ section of the graphic at the top of the post. I like that about Forbes, too.
So, let’s see what Forbes had to say:
I’d rate the chances that AEBi have the universal cure for cancer smaller than being struck by lightning whilst simultaneously being hit by a meteorite, seconds after finding out you’ve won the lottery.
https://www.forbes.com/sites/victoriaforster/2020/01/20/a-year-ago-an-israeli-research-group-said-they-would-cure-cancer-within-a-year-did-they-do-it/#39436f545e8d
I’m going to go out on a limb, here, and say that I think that Forbes has some reservations about MuTaTo being the cure for cancer…
And I tend to agree with Forbes, I don’t think that MuTaTo will be a cure for cancer. Not least because the discovery of the Cardiff Killer T Cell is a much simpler variation on a related theme.
That said, I hope I’m wrong about MuTaTo. I’m glad that AEBi is continuing with their research and I wish them all the best with their endevours.
The more that I’ve looked into this, the more I’ve come to the conclusion that the overblown hype surrounding MuTaTo was little to to with the staff at AEBi. The hype, rather, was generated by sensationalist reporting and click-bait culture within media.

By Gilles San Martin – originally posted to Flickr as Male human head louse, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=11208622
You’ve got to have those likes and shares if you’re going to sell advertising space! I mean, who’s really got time to read an article all the way to the end, much less conduct some fact checking?! And if the hopes and dreams of vulnerable cancer patients are dashed along the way? Well, that’s just the cost of doing business!

2 thoughts on “Can MuTaTo Cure Cancer, as Promised?”
A more realistic way cancer will probably be tackled will be by better diagnostics so it can be caught early in its development. An obvious example would be solid cancers such as pancreatic cancer. This sounds like a more feasible option than a “cure” and should occur much sooner than a “cure”.
Hi Phil,
I agree that better diagnostic tools would a great help. I’m just not sure how much better scans can get.
In the process of getting the diagnosis of my latest recurrence, I had the full range of scans that are available. The quickest was the CT scan, which scanned me from neck to groin, but wasn’t detailed enough to see the 35 mm tumour in my liver. It also came with a fairly significant dose of radiation.
The MRI scan was accurate enough to find the cancer, but took more than 30 minutes (about 5 times as long) just to scan my liver. At least there’s no radiation issue. Even this, though, is unable to accurately identify masses smaller than about 5 mm.
The PET/CT scan involved a lot of both time and exposure to radiation and, while it identifies cancerous areas, is often prone to false positives. Two of the four PET scans I’ve had have needed follow-up scans for false positives.
If a way could be found to make MRI scans faster and cheaper, I think that would be a great help. But, currently, an MRI scan from neck to groin would take well over an hour. How many people can lie perfectly still for that long? How many machines and radiographers would be needed to do MRI scans on all patients?
I’m just not sure how much more accurate a non-invasive scan can be than what we currently have.
So, while I agree that faster, cheaper and more accurate non-invasive diagnostic options would be ideal, I don’t see it happening any time soon.
In the meantime, I’m grateful that research is continuing in all available directions.
Take care,
Paul